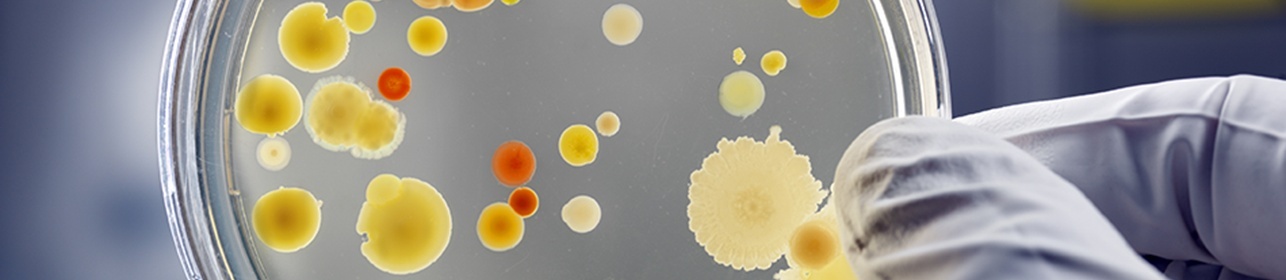
Technology of BACTOSTAT™ differs from silver-based or metal complexes or biocides leaching technology and coating technology.
The advantages of BACTOSTAT™ at a glance :
- Bacterial repellent : up to 99.99% under ASTM 66122 test method on S.Aureus.
- Antiviral activity : up to 90% under ISO 21702 test method on Omicron BA.2
- Health-eco-friendly: nil route of human, soil and aquatic exposure to leachable, releasable and inhalable additives
- Biocide-free: do not at all contain biocides or allergenic organics(triclosan, paraben, chlorhexidine, biguanide, etc.)
- Metals-free : free of Ag. Cu. Zn., metal complexes and/or other metals
- No nanoparticles & No migration: safe for humans, safe for animals and safe for the environment
- No bio-threat : no superbug
- Durable protection: non-leaching, no coating, long-lasting bacteria repellent functions along with lifetime of the polymer materials
- Food-Contact-Safe: Non-toxic, US-FDA and (EU)10/2011 compliant
- BPA-free, Phthalates-free
Test Result of BACTOSTAT™ Antimicrobial Polymers